
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
Methylene cyclopropane compounds (MCPs) are molecules with high tension but relatively stable at room temperature. Even in certain natural products, such as hypoglycin A and methylenecyclopropylglycine B, they contain methylene cyclopropane structure (Figure 1). Due to the presence of double bonds outside the ring, the three-membered ring is subject to greater spatial tension. By comparing the bond length and bond angle of methylene cyclopropane and cyclopropane, it can be seen that the bond length and bond angle have been increased (Figure 1).
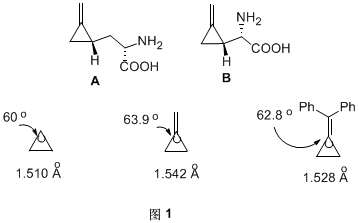
The high tension of methylene cyclopropane makes it prone to a series of reactions such as addition, ring opening, rearrangement, etc. that help to relieve ring tension, thus establishing its important position in organic synthesis. It must be noted that the reactivity of the cyclic compound is not directly proportional to its tension energy, but depends on the degree of tension energy released by the compound.
In the past few decades, chemists have been devoted to studying various reactions of methylene cyclopropane catalyzed by transition metals. Especially in the past decade, methylene cyclopropane has been widely used in various organic reactions And in total synthesis. Under the catalysis of transition metals Rh, Ru, Pt, Pd, etc., chemists found that methylene cyclopropane mainly has the following three ring-opening modes (Figure 2):

Shi Min's research group of the State Key Laboratory of Metal Organic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences conducted a systematic study on the ring-opening tandem reaction of methylene cyclopropane compounds. In 2002, it was discovered for the first time that under the action of metal Lewis acid (LA) or Brønsted acid catalyst (BA), proximal ring-opening can occur to obtain a novel structure compound. Under the transition metal catalysis, in addition to the remote ring opening, when one of the two R substituents is a hydrogen atom, a carbene-like rearrangement reaction occurs to obtain a cyclobutene compound. Under the action of Brønsted base (BB) such as LDA or nBuLi, a series reaction of carbon anions and various electrophiles can be generated (Figure 3).
Related research work was published in Org. Lett. 2002, 4, 2145 .; Org. Lett. 2003, 5, 579 .; Org. Lett. 2003, 5, 1415 .; Adv. Synth. & Catal. 2003, 345, 963 .; Chem. Commun. 2004, 2878 .; Org. Bioorg. Chem. 2005, 3, 399 .; Chem. Eur. J. 2006, 12, 510 .; Org. Lett. 2006, 8, 625 .; J. Am. Chem. Soc. 2006, 128, 7430 .; Chem. Eur. J. 2007, 13, 862 .; Org. Lett. 2007, 9, 117 .; Org. Lett. 2007, 9, 1303 .; Org. Lett. 2007, 9, 2405 .; Org. Lett. 2007, 9,4917 .; Org. Lett. 2007, 9, 5187 .; Chem. Commun. 2008, 2668 .; Organometallics 2009, 28, 5600 .; Dalton Transactions 2010, 39, 8829 .; Org. Lett. 2010, 12,116. Org. Lett. 2010, 12, 2606 .; Eur. J. Org. Chem. 2011, 7189, etc.

In the 2012 Acc. Chem. Res. (2012, 45, 641-652), the researchers summarized the new mode of reaction of methylene cyclopropane tension molecules, which can be constructed in one step by reacting with other compounds. Complex molecules (Figure 4).

The research work was supported by the Chinese Academy of Sciences, the National Natural Science Foundation of China, the Ministry of Science and Technology and the Shanghai Municipal Science and Technology Commission.
E-mel kepada pembekal ini

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.